D394VRG
Egida VR
Device Survival Probability
D3xxVRx_SURV
Loading...
| 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | 6 yr | 7 yr | 8 yr | 9 yr | 10 yr | 11 yr | 12 yr | at 145.0 mo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 100.0 | 100.0 | 100.0 | 99.9 | 99.9 | 99.9 | 99.8 | 99.8 | 99.7 | 99.7 | 99.7 | 99.7 | 99.7 | |||||||||
| % | 99.9 | 99.9 | 99.7 | 99.5 | 99.2 | 98.8 | 97.4 | 92.9 | 79.8 | 57.1 | 48.9 | 47.0 | 46.6 | |||||||||
| # | 25806 | 23987 | 22247 | 20577 | 19027 | 17454 | 15701 | 13107 | 8578 | 4306 | 2814 | 442 | 249 |
- Including Normal Battery Depletion – This curve includes devices that have reached at least 80% of expected longevity. This curve is most representative of clinical performance and how long the device will last.
- Excluding Normal Battery Depletion – This is the malfunction free survival curve.
Product Characteristics
| Generator Type | ICD |
|---|---|
| NBD Code | VVE-VVIR |
| Max. Delivered Energy | 35 J |
| Connector Style | Cx Style |
|---|---|
| Serial Number Prefix | PXO |
| Mass | 68 g |
| Volume | 37 cc |
| Waveform | Biphasic |
| X-Ray ID | PSI |
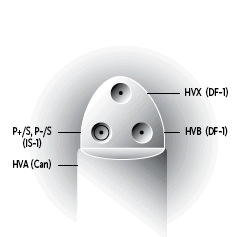
Cx Style
Estimated Longevity
| Charging Frequency | 100% Pacing | 50% Pacing | 15% Pacing | 100% Sensing |
|---|---|---|---|---|
| Monthly | 4.3 | 4.7 | 4.9 | 5.0 |
| Quarterly | 6.7 | 7.5 | 8.1 | 8.4 |
| Semi-annual | 7.8 | 8.9 | 9.8 | 10.3 |
Longevity estimates based on the following device usage. Pacing Mode VVI; Ventricular Lower Pacing Rate 60ppm; Ventricular Pulse Width 0.4ms; Ventricular Pulse Amplitude 3V; Ventricle Impedance 510ohms; Ventricle Sensing Rate 70bpm; EGM prestorage OFF; Optivol ON; Charging frequency assumes maximum energy charge and includes therapy shocks and capacitor formation. Hide this content
Distribution Data
| US Market Release | |
|---|---|
| CE Approval Date | 2011-01-12 |
| Registered USA Implants | |
| Estimated Active USA Implants | |
| Normal Battery Depletions |
| US Market Release | |
|---|---|
| CE Approval Date | 2011-01-12 |
| Estimated WW Distribution | 8793 |
| Normal Battery Depletions | 11 |
EOL Indicator
From the point that the RRT is set, the pacemaker will operate for approximately three months for typical pacemaker configurations during the normal operating life.
Data as of November 20, 2024