Increased Potential for Reduced Energy or No Energy Delivered During HV Therapy When Programmed AX>B (2023)
Cobalt™ XT, Cobalt and Crome™ ICDs and CRT-Ds
Original Date of Communication:
May 2023
View specific models that this applies to
Devices managed with an updated SmartSync tablet (device application software 8.1.0 or higher) are no longer in scope of the May 2023 communication.
STATUS UPDATE - FEBRUARY 2024
As of 20 February 2024, Medtronic has identified 35 devices (representing 0.0035% of devices distributed worldwide) that experienced the issue described in the communication. No permanent harms or deaths have been attributed to this issue. When programmed B>AX, the occurrence rate remains in line with the historic non-advisory population as described in the original communication. To support the patient management recommendations in the May 2023 communication, Medtronic reviewed more than 680,000 shock events that occurred in over 228,000 unique devices.
Medtronic is releasing a software update that aligns SmartSync tablets with the programming recommendations. This update is available in the United States, Europe, Australia and New Zealand and will be made available in other geographies and on other programming platforms pending regulatory approvals. Devices managed with an updated SmartSync tablet (device application software 8.1.0 or higher) are no longer in scope of the May 2023 communication.
The overall reliability of these devices remains high. The graph below shows device survival probability inclusive of all confirmed malfunctions.
| 1 yr | 2 yr | 3 yr | |
| % | 100.0% | 100.0% | 99.9% |
| # | 76,347 | 44,532 | 12,119 |
ORIGINAL COMMUNICATION - MAY 2023
This communication advises physicians of a rare potential for reduced- or no-energy output during high voltage (HV) therapy (typically 0-12J) in implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) manufactured with a specific (glassed) feedthrough, including currently available ICDs and CRT-Ds. Through 10 April 2023, Medtronic has identified 27 devices from approximately 816,000 devices (representing 0.003%) distributed worldwide that have experienced a reduced- or no-energy HV therapy due to the issue described in this letter. There have been no deaths due to this issue in the glassed feedthrough device population.
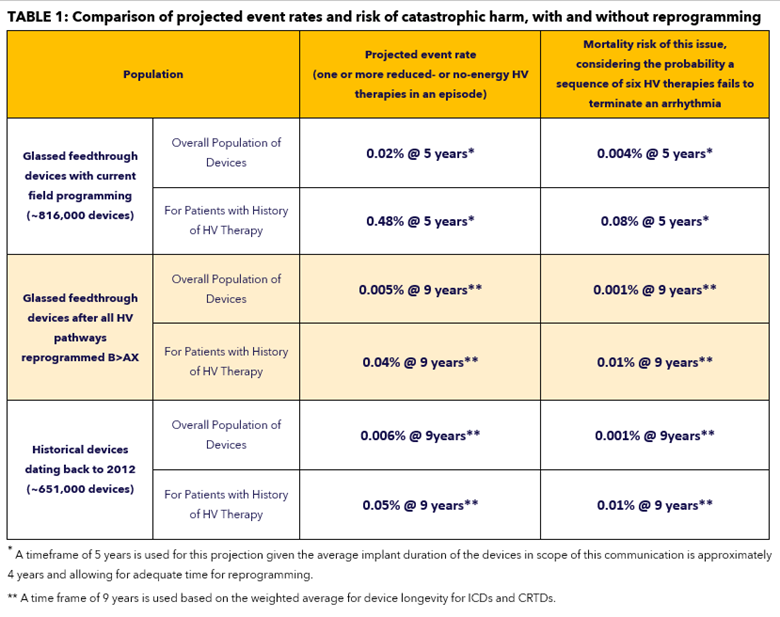
With current field programming, devices with a glassed feedthrough may experience increased potential (projected to be 0.02% at 5 years) for reduced- or no-energy output during HV therapy. In some cases, a persistent 50% drop in all pacing lead impedances may be displayed; lead function, however, is not affected. See Issue Details below for further information on root cause, projected rates, risks, and potential harms.
A broader analysis was conducted to determine the incidence of device related reduced- or no-energy HV therapy events outside of the above population, with implants dating back to 2012. Using these historical observed events, projected rates for this population of devices is 0.002% at five (5) years and 0.006% at nine (9) years. This analysis identified two deaths from the historical population in which there was evidence suggesting a device-related reduced- or no-energy HV therapy occurred.
Should this issue occur, pacing, sensing, episode detection, anti-tachycardia pacing (ATP) therapies, battery longevity, and telemetry remain functional. When devices in the glassed feedthrough population are programmed exclusively in the B>AX configuration, the potential for a reduced- or no-energy HV therapy is 0.002% at five (5) years and 0.005% at nine (9) years, comparable to historical device performance.
PATIENT MANAGEMENT RECOMMENDATIONS:
Medtronic recognizes that each patient requires unique clinical considerations. Based on internal investigation and external consultation with our Independent Physician Quality Panel (IPQP), Medtronic provides the following guidance:
-
Prophylactic device replacement is NOT recommended.
- The risk of mortality for patients after reprogramming is 0.001% at 9 years and is less than the risk of patient mortality due to complications associated with device replacement (0.032% - 0.043%1,2,3).
-
Program all HV therapy pathways B>AX in all therapy zones to minimize the risk for this issue.
- Note: Using “Get Medtronic Nominals” will require manual reprogramming of Rx5 and Rx6 to B>AX for all ventricular therapies.
- For patients remotely followed via CareLink, your Medtronic representative will provide a report to assist with identifying patients who may have one or more HV therapy pathways programmed AX>B. You may contact your local representative to obtain an updated copy of the report at any time.
-
Prioritize reprogramming patients who have both a history of HV therapy and Rx1 programmed AX>B.
- Rx1 provides the greatest statistical likelihood to resolve an arrhythmia, and therefore it is important to minimize risk of a reduced- or no-energy HV therapy in the first sequence.
- For remaining patients with AX>B programming in any HV therapy sequence, schedule the next follow-up for in-clinic reprogramming, with the appropriate discretion, to minimize potential for reduced- or no-energy HV therapies to occur.
-
Per standard practice, check tachyarrhythmia episodes to determine effectiveness of therapies that have been delivered.
- Instruct patients to contact the clinic if they receive HV therapy or hear an audible tone coming from their device.
-
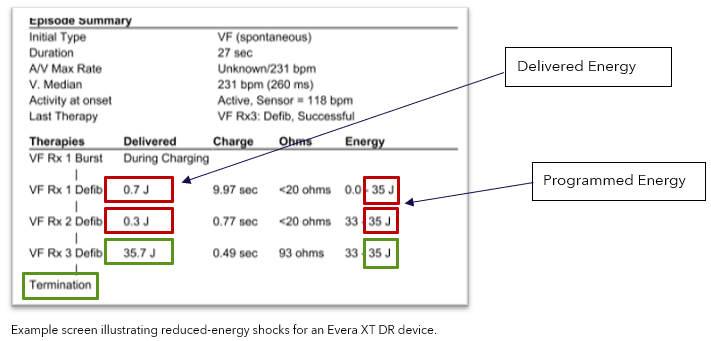
Verify delivered energy is consistent with programmed energy in the Episode Summary.
Note: The image below highlights an episode experiencing intermittent reduced HV therapies (red boxes).
- After an SCP event, pacing, sensing, episode detection, and anti-tachycardia pacing (ATP) therapies are not impacted; additionally, HV charging, battery longevity and Bluetooth telemetry are not impacted.
-
Contact Medtronic Technical Services (1-800-929-4043) or your local representative if one of the following is observed as these may be an indication of either a device or lead-related issue:
- Reduced- or no-energy HV therapy is displayed in Episode Text (regardless of programmed pathway)
- A persistent drop of approximately 50% in RA, RV and LV pacing lead impedance measurements as this may be an indication of increased potential for a future reduced- or no-energy therapy.
ISSUE DETAILS:
There is an increased potential for a reduced- or no-energy HV therapy in the AX>B configuration when all the following conditions are met:
- The device has a glassed feedthrough (manufactured after July 2017).
- There is significant separation of the layers of insulation materials in the feedthrough components of the device header.
- An unintended current pathway forms within the void created by the insulation separation, capable of conducting high levels of current during HV therapy.
When an unintended current pathway is detected during HV therapy, the Short Circuit Protection (SCP) feature may trigger (see Appendix A). This behavior can be intermittent; both full-energy and reduced-energy HV therapies within the same episode have been observed. SCP events may also be lead related; for both lead-related and device-related unintended current pathways, the defibrillation waveform is truncated early in the energy delivery sequence, resulting in reduced or no energy being delivered (~0-12J).
RATES OF OCCURRENCE FOR DEVICE-RELATED REDUCED- or NO-ENERGY HV THERAPY:
Through 10 April 2023, 27 devices with glassed feedthroughs have been identified as experiencing a reduced or no-energy HV therapy (0.003% out of 816,000 distributed devices). Of these, 26 were in devices with an AX>B delivered pathway. Based on an analysis of patients with a glassed feedthrough device and with a history of HV therapy, the observed rate for this issue is 0.03%. See Table 1 below for projected rates of occurrence and risk for harm.
Potential harms related to reduced- or no-energy HV therapy include failure to terminate an arrhythmia, which could lead to death, as well as complications associated with device replacement and/or unnecessary lead replacement if the reduced- or no-energy HV therapy is erroneously attributed to a lead failure.
Medtronic is updating Instructions for Use, HV therapy nominals and the programmer interfaces for these device models to be consistent with information in this letter. Medtronic will release additional information once the necessary regulatory approvals have been received, as applicable in the local region.
APPENDIX A – ADDITIONAL DETAILS ON SHORT CIRCUIT PROTECTION (SCP)
Short Circuit Protection (SCP) is a safety feature that can only occur during high voltage (HV) therapy. SCP is designed to truncate energy delivery to protect the device when an unintended current pathway is detected during a shock. An SCP event can occur when an unintended current pathway develops either in the lead or in the device. Contact Medtronic for additional guidance if you believe an SCP event occurred.
Programming all high voltage delivery pathways to B>AX will minimize the effect of an unintended current pathway in the device header (e.g., feedthrough), thereby minimizing the potential for an SCP event. Comparing the programmed energy to the delivered energy in the Episode Text for a treated episode can be used to identify SCP events. The lead impedance in the Episode Text will also display <20 ohms. Refer to the sections below on how to identify if an SCP event has occurred.
For Cobalt/Crome ICD and CRT-D devices:
Identifying SCP events during high voltage (HV) therapy delivery: When an SCP event has occurred, the device will issue an RV Defibrillation Lead Impedance CareAlert. Audible and wireless alerts are issued, if enabled. These devices are shipped with alerts enabled and are nominally active once implant detection completes.
A CareAlert will occur simultaneous with HV therapy delivery and will specifically report “RV Defib Lead Impedance 0 Ω” with the same time stamp as the therapy delivery. The alert condition is displayed in the CareAlert Events log (Data >> CareAlert Events). The Quick Look Observations will also display “Alert: RV defib lead impedance warning on Mmm/DD/YYYY.” SCP events are not reflected in the long-term lead impedance trends.
Following an SCP event in devices with both the SVC Coil and Active Can enabled, the device will turn off the SVC coil. If the SCP event is caused by a lead integrity issue involving the SVC coil, turning off the SVC coil can allow any subsequent shocks to be delivered using the RV Coil -to- Active Can vector. The programmed therapy parameters will indicate the SVC Coil has been automatically disabled. The SVC coil will remain off until it is turned back on by the clinician.
If an SCP event occurs during a manual shock delivery, there will be no episode data. For manual shock deliveries, review the shock impedance and the delivered energy from the Last High Voltage Therapy section on the Battery and Lead Measurement screen of the programmer.
1Tarakji KG, et al. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. The New England Journal of Medicine. 2019; 380(20):1895-1905.
2Medtronic Data on File. MDT2260884-CRHF CIED Infection Report; Agile: MDT2260884, Version 2.0, 11/02/2015.
3Birnie D, et al. Complications associated with defibrillation threshold testing: The Canadian experience. Heart Rhythm. 2008; 5(3):387-90.