Ensuring the Accuracy of Battery Longevity Estimates
View specific models that this applies to
Purpose of This Information
This article is intended to help the clinician understand how Medtronic estimates CRT-D, ICD, and IPG device longevity and Medtronic’s performance against these estimates.
Device Longevity and Battery Depletion
The device service life ends when the usable battery capacity is depleted. The time to battery depletion depends on three factors:
- The amount of electrical energy expended in providing therapy to the patient
- The amount of energy consumed by the electronic circuitry to perform the functions of the device (e.g., operating the microprocessor, telemetry, memory, and charging component)
- The energy capacity of the battery
Medtronic has developed a statistical model for device longevity that accounts for each of these factors, and has validated the model with real time clinical performance. During the development of its products, Medtronic engineers characterize device longevity using this model. Testing begins during development and continues after market release to ensure the accuracy of device longevity estimates.
Using Survival Curves to Assess Longevity
The survival curves in the Product Performance Report represent the composite experience of thousands of devices over a wide range of programming options and patient use conditions. While the curves are useful for understanding the overall performance of a population of devices, they cannot be used to accurately predict the longevity of a specific device in a specific patient. To get a longevity prediction for a specific device, the longevity model must be used. The model is available by contacting Medtronic’s Technical Services Department.
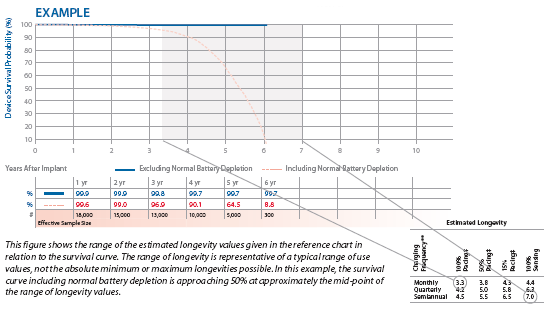
Because the survival curves are an aggregate result, the Reference pages in the Product Performance Report include several longevity estimates for a range of use conditions. These longevity estimates are mean values calculated for the parameters given. This range of longevity estimates can be compared to the survival curve including normal battery depletion to assess the overall clinical performance of a device model against the original longevity estimates.
If most of a device model’s population is being used at nominal parameters and conditions, the time at which the survival curve including normal battery depletion equals 50% should approximate the midpoint in the range of longevity estimates.
If devices tend to be used at conditions that consume more or less energy than nominal, then the time at which the survival curve equals 50% should tend toward the lower or higher end of the range of longevity estimates, respectively.