Potential for Shortened RRT-to-EOS in Subset of ICDs and CRT-Ds
Subset of ICDs and CRT-Ds
Original Date of Communication:
February 2021
View specific models that this applies to
STATUS UPDATE - APRIL 2024
As of 18 March 2024, approximately 167,713 devices susceptible to this issue are estimated to still be active worldwide. Observed rate of occurrence is 0.16% and projected rate for the affected population of devices remains 0.22%. Devices with higher pacing outputs and high pacing percentages (e.g., CRT-D devices) have the lowest probability of occurrence (refer to Appendix A of the original communication for further details – see below). No permanent patient harms have been reported due to this issue.
ORIGINAL COMMUNICATION – FEBRUARY 2021
In February 2021, Medtronic informed physicians of a potential issue for a subset of Implantable Cardioverter Defibrillators (ICDs) and Cardiac Resynchronization Therapy Defibrillators (CRT-Ds). Medtronic has identified that a small percentage of implanted cardiac devices, from a well-defined subset, may experience a shortened Recommended Replacement Time (RRT) to End of Service (EOS) interval following an earlier-than-expected RRT observation. The subset of ICDs and CRT-Ds affected by this issue were last implanted in February 2019 and manufactured with a specific battery design that is no longer being distributed.
We have received no reports of permanent harm to patients as a result of this issue.
Approximately 339,900 devices susceptible to this issue are estimated to still be active worldwide. Through 4 January 2021, confirmed events (observed rate 0.07%) have involved a rapid drop in battery voltage ranging from days to months, with unexpected RRT as one of the primary reported observations. For those devices in which RRT triggered earlier than expected, the median time from RRT to the EOS observation was 14 days. In a small number of the cases, no output/no telemetry was reported prior to device replacement. Medtronic projects approximately 0.22% of the affected device population may experience this issue during their service life.
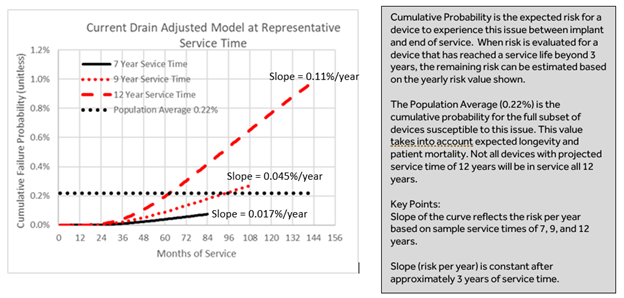
The rapid depletion is caused by a latent shorting mechanism involving lithium plating, resulting from a thermal gradient between the anode and cathode components of the battery. Devices with higher pacing outputs and high pacing percentages (e.g. CRT-D devices) have the lowest probability of occurrence (refer to Appendix A – see below). Conversely, devices with low current drain (evidenced by a longer overall service time from implant to RRT) have a higher probability of experiencing this issue. Importantly, the probability of this issue developing is constant after approximately three years of service time.
Patient Management Guidance
We realize that each patient requires unique clinical considerations. In consultation with our Independent Physician Quality Panel (IPQP), Medtronic recommends the following:
-
Continue normal follow-up per local clinical protocol.
- Recognize that patients who require significant pacing support and high voltage therapy have the lowest risk for this issue - See Appendix A for additional details.
- Where possible, take advantage of the CareLink™ home monitoring system and the wireless low battery voltage CareAlert.
- The low battery voltage audible alert is shipped On with high-urgency tones; Remind patients to contact their clinic if they hear an audible alert, particularly since patients may be opting to delay clinic visits due to COVID-19 guidance.
- Inform a Medtronic Representative of any unexpected device behaviors.
- Be aware that the inability to interrogate the device, or to transmit data, may be an indicator that the device has experienced this issue.
-
If unexpected RRT is observed, prompt replacement of the device should occur commensurate with the underlying clinical situation of the patient:
- For non-pacing dependent patients or for primary prevention ICD patients, replacement within 1 week of an unexpected RRT notification is recommended.
- For pacing dependent patients, immediate replacement is recommended following an unexpected RRT notification.
Note: For all patients, this issue can also manifest as an unexpected change in the remaining longevity estimate that cannot be attributed to programming changes, or changes in use conditions.
Medtronic medical staff in consultation with the IPQP recommends against prophylactic replacement due to the low rate of occurrence and the low potential for permanent harm when prompt replacement occurs in response to an unexpected RRT.
Patients and clinicians may determine if a specific device is affected by looking up the serial number on Medtronic’s Product Performance website: http://wwwp.medtronic.com/productperformance/
APPENDIX A
The table below provides a comparison of sample use conditions and their associated, projected service time (Implant to Recommended Replacement Time), along with their cumulative and per-year risk of encountering rapid depletion due to a latent shorting mechanism in the battery. Devices with higher pacing outputs and high pacing percentages have the lowest probability of occurrence. There have been no reports of permanent harm to patients as a result of this issue.
Probability (Risk per Year) of Rapid Depletion due to this Issue as a Function of Service Time
|
Projected Service Time * (based on sample programmed settings and use conditions) |
Projected Risk per Year & Total Cumulative Risk at end of service time++ |
Notes/Example |
|
12-year service time |
0.11% per year, 0.98% cumulative |
VR ICD patient with 0% pacing and no shocks delivered |
|
10.25-year service time |
0.070% per year, 0.50% cumulative |
VR ICD patient with 50% pacing history and two (2) or fewer shocks per year |
|
9-year service time |
0.045% per year, 0.27% cumulative |
DR ICD patient with little-to-no pacing history (e.g. 10%AP, 25%VP, and two (2) or fewer shocks per year) |
|
8.25-year service time |
0.033% per year, 0.18% cumulative |
DR ICD patient with complete heart block (10% AP and 100% VP, and two (2) or fewer shocks per year) |
|
7-year service time |
0.017% per year, 0.075% cumulative |
CRT-D patient with 15% AP, 90% RVP, 100% LVP, and two (2) or fewer shocks per year |
|
* Assumes current drain remains stable throughout life of device (i.e. No change in remaining longevity due to reprogramming or changes in use conditions) |
++ Per annum risk of issue becomes constant after approximately 3 years of service time. Cumulative risk = early risk plus annual risk over the projected service time. |
A output = 1.5V, 0.4ms, 500 ohms RV output = 2.0V, 0.4ms, 500 ohms LV output = 2.5V, 0.4ms, 500 ohms Average pacing rate = 75 bpm |