 |

|  | What is Neurostimulation ?
Overview
of neurostimulation
Neurostimulation systems use an implanted lead to deliver low-voltage electrical
stimulation to selected nerves or anatomic structures. Neurostimulation
is divided into subcategories based upon the type of nerve that is being
stimulated. Spinal cord stimulation (SCS) involves stimulation of the dorsal
column of the spinal cord by placing electrodes in the space above the spinal
cord. Neurostimulation can also be used on the peripheral nerves by stimulating
a specific nerve branch in the affected limb. This site-specific electrical
stimulation inhibits or blocks the sensation of pain in a targeted region
of the body.
The mechanism
of action for neurostimulation
The mechanism of action for neurostimulation is based upon the use of electricity
(Melzack R, Wall PD. Science 1965; 150:971-979). The use of electricity
for pain relief is based on the gate control theory, which suggests that
a metaphorical 'gate' exists in the spinal cord that allows or prohibits
the transmission of pain signals to the brain. |
Neurostimulation systems
Neurostimulation systems consist of three basic components that generate
and deliver electricity
in the form of short bursts or pulses to large nerve fibres in the dorsal
column or the periphery: |
- Power source
- Extension
- Surgical or percutaneous leads
|
|
Power source
The neurostimulator's power source is a battery that generates electrical
pulses to create paraesthesia. There are two types of power sources:
- Internal - Implantable pulse generator (IPG)
- Internal - Implantable pulse generator (IPG)
- External - Radio frequency (RF) system
|
|
Implantable pulse generator (IPG) power
source
In an IPG system the entire system, including the battery, are implanted
within the patient's body. IPG systems are capable of meeting the needs
of most patients with chronic pain,except those with very high expected
energy consumption. For these patients an RF system is recommended. |
Radio frequency (RF) power source
An RF system consists of two components: |
- A transmitter and antenna that are worn externally
- A receiver that is surgically implanted
|
| The external transmitter sends RF signals through
the skin to the implanted receiver, which is surgically placed under the
skin. The receiver processes the RF signals from the transmitter and generates
electrical pulses for neurostimulation. |
|
Generally patients prefer IPG systems because the
totally implantable IPG systems are seen as more comfortable, convenient
and cosmetically appealing than external neurostimulators. In addition,
unlike RF systems, IPG systems do not cause skin irritations. For these
reasons, patients will often be more compliant, and overall therapy will
be more effective. Patients using IPG systems may also have greater ease
in daily activities, such as working, exercising and sleeping.
Extension
The extension cable connects the power source to the lead. Using an extension
rather than a lead connected to the IPG gives additional benefits in terms
of being easier to handle in case of revisions and offering more comfort
for patients.
Leads and electrodes
The lead is a polyurethane-encased wire connected to a set of electrodes.
The lead conducts electrical pulses from the extension to the electrodes,
which deliver the pulses to large nerve fibres in the dorsal column or
the periphery. Electrodes are fixed at the end of the lead, usually in
groups of four. Optimal lead positioning always requires the cooperation
of the patient and is the key to success with this therapy.
|
| Paraesthesia
coverage |
Area of stimulation |
| Upper limb |
C3-C5 |
| Precordium |
T1-T2 |
| Lower back and lower limb |
T8-T9 |
| Foot |
T12-L1 |
|
For spinal cord stimulation (SCS), leads are placed
in the epidural space (between the vertebrae and the dura matter) so that
the electrodes are close enough to the dorsal horn to stimulate specific
large nerve fibres. Leads can be implanted into the spinal column in one
of two ways:
|
- Percutaneously through a needle
- Surgically
|
|
The functioning neurostimulation system provides
a flow of electrical pulses from the power source through the extension
and lead to the electrodes. The electrical pulses are then conducted into
the dorsal column of the spinal cord or the periphery to produce paraesthesia.
Benefits of Neurostimulation
|
| SCS treatment of chronic back and leg pain associated
with FBS: an overview |
- Timely treatment of FBS patients provides best
results
- reducing the delay between spinal surgery and implantation from
12 years to 3 years increases the success rate from 9% to 94% [2]
- In a literature review of 39 articles,
50-60% of patients had 50% or more pain relief after SCS [3]
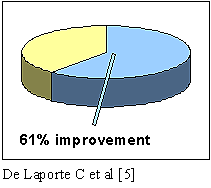
- SCS also improves functional status in a significant
number of patients, with a 31% return-to-work rate [4] and up to a 61%
improvement in activities of daily
living [5]
- SCS reduces the need for analgesics from between
40-84% [2,6]
|
 |
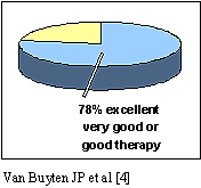
- In a study in 153 patients with a mean follow-up
of 3.6 years, 78% of patients judged their therapy to be excellent,
very good or good [4]
- In 50 patients
with FBS, averaging 3.1 operations, successful outcome was recorded
in 53% of patients at 2.2 years, and in 47% of patients at 5 years [7]
- A randomised comparative
study of SCS versus re-operation found that [8]:
- 67% of patients
who had been randomized to re-operation opted for cross-over to SCS,
whereas
- only 17% of patients
treated with SCS crossed over to surgery after 6 months of follow-up.
|
 |
SCS for the treatment
of low back and/or leg pain and extremity pain
- In a prospective study of SCS in patients with
intractable leg pain, a success rate of 53% was reported at six weeks
[6]
- In a multicentre study of SCS for the
relief of chronic back and extremity pain in 70 patients, pain was successfully
managed in 56% of patients after 1 year of follow-up [9]
- In 30 patients treated with SCS for low back
pain and radicular pain after multiple failed back surgeries, 75% continued
to report at least 50% pain relief after 34 months of follow up [10]
SCS: dual stimulation studies
- The ability to add a second lead if necessary
may improve success in a wider range of patients, including patients
with difficult to treat conditions such as axial low back pain
- In a trial of 41 patients with chronic, intractable
low back pain, 69% were satisfied with treatment and 75% would repeat
procedure if they had known the outcome before the operation. [11]
- 90% of patients required the addition of a second
lead.
- In a trial in 21 patients with FBS, patients'
pain scores substantially decreased, and 76% of patients' indicated
satisfaction with dual lead SCS treatment after nearly 40 months of
follow-up. [12]
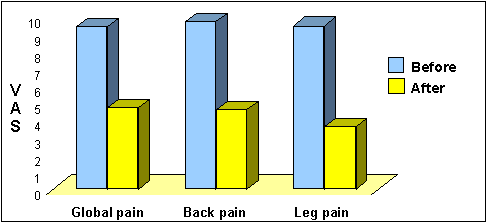
- In a trial in 23 patients with complex back
and leg pain, and a history of back surgery, patients visual analogue
scores improved by a mean of 58% for back pain, and 45% for leg pain
with dual lead SCS treatment. [13]
|
|
Dual lead SCS provides good low
back and leg pain relief
VAS pain ratings before and after
dual lead SCS implants (n=17) [12]
|
|
SCS: Cost effectiveness
- SCS has been shown to be a more cost effective
treatment for FBS than re-operation [14]
- Surgery success rates decrease
with the number of re-operations. [7]
- The actual mean cumulative cost for SCS therapy
for a 5-year period was $29,123 per patient compared with $38,029 per
patient for conventional pain therapy. [15]
|
|
- In a prospective trial of SCS compared with
re-operation, not only was there a clinical advantage for SCS, but this
therapy was also the most cost-effective over 3 years of follow-up.
[1]
- A cost analysis model of patients with FBS
indicates that SCS therapy can lead to cost savings over conservative
treatment by reducing patients' needs for medical care. [16]
- On average, given current screening and efficacy
rates, SCS therapy pays for itself in 5.5 years; in patients who respond
well to SCS treatment, the therapy pays for itself in 2.1 years. [16]
|
References
1. North RB et al, J Neurosurg 1997;86:abstract#748
2. Kumar K et al, Surg Neurol 1998; 50:110-21
3. Turner JA et al. Neurosurgery 1995;37:1088-1096
4. . Eur J Pain 2001;5(3):299-307
5. De Laporte C et al. Pain 1993;52:55-6
6. Ohnmeiss DD et al. Spine 1996; 21: 1344-1351
7. North RB et al;. Neurosurgery 1991;28:692-9
8. North RB et al. Stereotact Funct Neurosurg 1994;62:267-72
9. Burchiel KJ et al. Spine 1996;21:2786-94
10. Leveque JC et al. Neuromodulation 2001;4(1):1-9
11. Ohnmeiss DD, Rashbaum RF. Spine J 2001;358-363
[dual leads were required for these patients]
12. Van Buyten JP et al. Neuromodulation 1999;4:258-265
13. Milbouw G, Van Buyten JP et al. 9th Worldwide pain conference 2000 San Francisco
14. Carette S et al. N Engl J Med 1991;325:1002-1007
15. Kumar K et al. Neurosurgery 2002;51:106-116
16. Bell GK et al. J Pain Sympt Manage 1997;13:286-95
|


