 |

|  | Chronic Back & Leg Pain
Definition
Failed Back Syndrome (FBS) is clinically defined as persistent or recurrent
pain, mainly in the region of the lower back and legs, after lumbosacral
spine surgeries that are technically and anatomically successful. |
Epidemiology
Approximately 5% of people experience a new episode of low back pain each
year, and over a lifetime, 60%-85% of people will experience an episode
of low back pain (Svensson 1989; Frymoyer 1991; Papageorgiou 1995; Cassidy
1998). One-third of those patients experiencing
low back pain go on to develop chronic or recurrent low back pain (Hakelius
1970; Weber 1983).
Half of all spinal surgeries performed are lumbosacral (Segal 1998). Failed
back surgery occurs
in approximately 30% of these patients, in which "failure" is
defined by persistent or recurrent pain, with and without low back pain,
after anatomically successful surgery (Segal 1998; North 1994; North 1991a). |
Economic concerns
FBS sufferers represent a huge economic concern. Of more than 300,000 lumbosacral
surgeries performed annually in the U.S. (Segal 1998), approximately 100,000
new cases of FBSS are expected each year (Segal 1998; North 1991). FBS also
has a high prevalence in Europe.
In Belgium, for example, approximately 2,400 new patients present with FBS
each year
(population 10 million). With a typical FBSS patient having undergone at
least one previous lumbosacral operation, repeated lumbosacral operations
are successful in only 20%-30%
of patients, suggesting a worsening of their condition, in spite of, or
because of, surgery
(North 1991a; Fritsch 1996). Accordingly, FBS poses a huge therapeutic challenge. |
Symptoms
Patients with FBS may suffer from recurrent disc herniation, lumbosacral
postoperative fibrosis and/or arachnoiditis, epidural scarring, an injured
nerve root, dorsal compartment syndrome and/or lateral spinal stenosis.
Pain may arise from viscera, blood vessels, nerves, bones of the spine and
pelvis, muscles and/or joints. In most cases, a specific cause of the pain
associated with FBS cannot be identified. Physicians have even reported
cases in which patients who were admitted for surgical treatment of FBS
have subsequently been defined as having pain of a different origin. |
Causes
Several factors may contribute to this syndrome, including selection of
inappropriate patients for surgery (Spengler 1980; Zucherman 1986; Fager
1980; Long 1988), surgical complications, irreversible nerve injury and
psychosocial problems. |
Pain
Pain associated with FBS is thought to encompass a mixture of neuropathic
and nociceptive elements. The condition always involves the dorsal horn,
consisting of central sensitisation and mainly sympathetic involvement.
Pain that is primarily in the leg is thought to involve nociceptive pain
or neuropathic pain, or in some cases, a mixture of both types of pain.
In contrast, back pain
is thought to be the result of degeneration of intervertebral joints or
pseudoarthrosis, or an infection or degenerative change at other vertebrae.
For FBS sufferers, the pain associated with their condition remains typically
refractory, difficult to control and unlikely to respond to further reconstructive
surgery, suggesting that other treatments, such as neurostimulation, should
be considered. |
Current treatments
Recent clinical studies have focused on refining existing therapies for
FBS and identifying factors that could influence outcome and improve patient
selection criteria. |
Conservative
Conservative treatment is standard for most patients with chronic low back
pain. Treatment consists of a combination of physiotherapy (rehabilitation
and symptomatic treatment), oral medication, analgesics, non-steroidal anti-inflammatory
drugs and corticosteroids. Treatment
for obesity or depression might also be necessary. Patients are encouraged
to engage in normal physical activity as much as possible to prevent debilitation
due to prolonged rest. |
Orthopaedic surgery
Orthopaedic surgery normally involves interbody fusion (intervertebral implant);
surgical resection of disc herniation; discectomy, with or without interbody
fusion; and removal of a stenosis by decompressive surgery, with or without
interbody fusion. In patients with FBS, surgery is not usually suitable.
The success rate of repeated lumbosacral operations is only 20%-30% (North
1991a; Fritsch 1996). Many patients with FBS require multiple lumbar surgeries
before reporting clinical success and relief from pain, although some patients
never achieve pain relief. Thus, re-operation should be considered only
for those patients with FBS in whom pain can be attributed to a surgically
remediable lesion. In other patients with FBS who have had anatomically
successful surgery but still experience pain, the use of neurostimulation
has proved a more effective treatment option (North 1991a, 1991b). |
Neurostimulation and
intrathecal drug delivery
Neurostimulation and intrathecal drug delivery are minimally invasive, reversible
treatments for pain associated with FBS, compared with the sometimes destructive
and costly procedures of spinal surgeries. Recent technological advances
in neurostimulation and intrathecal drug delivery show that these treatments
are highly effective, suggesting that they should be used earlier. Patients
with low back pain usually respond well to neurostimulation, and this is
often explored before intrathecal drug delivery. The best indication for
neurostimulation is neuropathic pain of peripheral or nerve root origin.
Failure of a neurostimulation trial could indicate predominantly nociceptive
pain and should prompt an intrathecal drug delivery trial. |
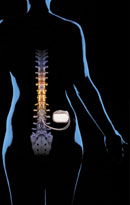
Neurostimulation
System |
Neurostimulation:
Neurostimulation is the treatment of choice for patients with FBS who suffer
from chronic neuropathic pain of a non-structural nature that is refractory
to conservative treatments. This therapy can be administered as either spinal
cord stimulation (SCS) or peripheral nerve stimulation (PNS). Of the two
techniques, SCS is much more widely used, with more substantial supporting
clinical data. SCS has been used as a method for relief of chronic pain
for more than 30 years, and involves electrical stimulation of the spinal
cord via implanted electrodes to produce paresthesia corresponding to the
areas of pain. The mechanism of action is still being elucidated. The most
commonly accepted theory is the gate-control theory of pain (Melzack and
Wall 1965). Other theories include inhibition of pain at the supraspinal
level and activation of central inhibitory mechanisms that influence sympathetic
efferent neurons. Conventional SCS techniques involve the implantation of
a single lead. One of the most challenging aspects of SCS has been the placement
of the electrode in a position that provides parasthesia to the painful
area without stimulation of the dorsal roots. |
| However,
technological advances using two leads have resulted in the potential to
steer the paresthesia and correct its coverage after implantation; for example,
by activating additional cathodes on the side with the weakest stimulation.
Other advances in the percutaneous placement of multiple electrode leads
have also yielded improved results. The development of neurostimulation
systems with more lead options and electrode spacing diversity has resulted
in improved clinical results and the ability to treat a greater number and
variety of patients. Recent clinical studies have shown promising results
for long-term pain relief with |
SCS
(Ohnmeiss and Rashbaum 2001; Kumar 1998; Kupers 1994; Burchiel 1996 and
Burchiel 1995 ). A prospective trial showed that SCS is superior to re-operation
as a treatment for FBSS (North 1994). Furthermore, at a recent advanced
workshop organized by the ECMT, Drs. Alo and Van Buyten agreed that the
availability of dual leads would reduce patient drop-out rates in those
patients with hard-to-treat pain conditions. SCS has also been shown to
improve performance
of activities of daily living (North 1993; De La Porte 1993). Importantly,
this treatment regimen is also cost-effective compared with re-operation
or long-term oral medication (Kumar 2002a; DeLissovoy 1997; Bell 1997; Kidd
1996). The most common issue arising from SCS treatment
is lead migration. This is generally managed by correcting lead placement.
Alternatively, by using an Octad lead, with 8 electrodes,lead migration
can be managed by reprogramming stimulation patterns to alternative electrodes
on the lead. |
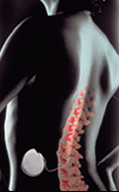 Intrathecal Drug
Intrathecal Drug
Delivery System
|
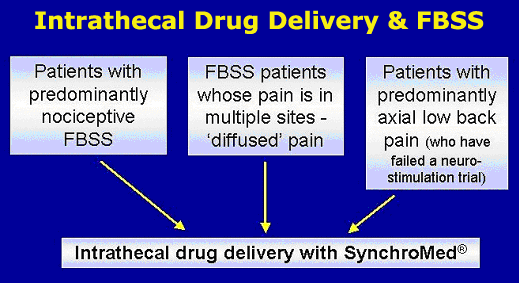
Intrathecal drug delivery:
Long-term opioid therapy is a viable option for FBS patients with pain
of a predominantly nociceptive nature,or
for those patients who have failed a neurostimulation trial. For patients
with predominantly nociceptive pain, SCS is less likely to relieve pain
than in those patients whose pain is neuropathic. Intrathecal drug delivery
involves the spinal administration of opioids via an implantable, programmable
drug delivery system. Site-specific delivery reduces
the opioid dose that must be administered, compared with systemic methods,
with the consequence that there are fewer side effects. A number of studies
have shown positive outcomes following treatment with intrathecal drug
delivery in patients with FBS (Anderson and Burchiel 1999; Paice 1996;
Winkelmuller 1996; Tuktak 1996).
In addition, programmable pumps allow for easy post-do titrationse. Utilising
patient controlled analgesia (PCA) permits the titration of the analgesic
dose to the analgesic requirements within physician-set limits and allows
for the management of breakthrough pain.Additionally, intrathecal drug
delivery has been demonstrated to be cost-effective for the treatment
of FBS (Kumar
2002b) in patients who fail a neurostimulation trial
|
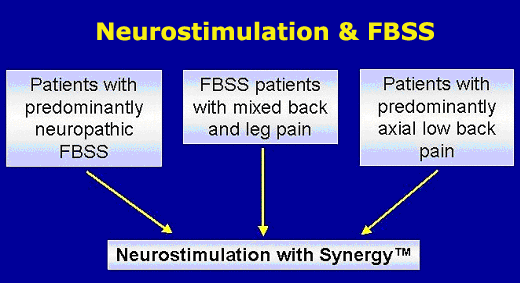
Treatment pathway for FBS
Neuromodulation is the treatment of choice for patients
with FBS who suffer from chronic pain of
a non-structural nature that is refractory to conventional treatment. |
Further reading
References:
|
- Anderson V,
Burchiel K. A prospective study of long-term intrathecal morphine in the
management of chronic
non-malignant pain.Neurosurgery 1999;44(2):289-300.
- Bell GK, et al. Cost effectiveness
analysis of spinal cord stimulation in treatment of failed back surgery
syndrome.J Pain Symptom Manag 1997;13:286-295.
- Burchiel K, et al. Prognostic
factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery
1995;36:1101-11.
-
Burchiel K ,et al. Prospective, multicenter study of spinal cord stimulation
for relief of chronic back and
extremity pain. Spine 1996;21:2786-94.
- Cassidy DJ. The Saskatchewan Health and Back Pain Survey: the prevalence
of low back pain and related
disability in Saskatchewan. Spine;1998;23:1860-7.
-
De La Porte C, et al. Spinal cord stimulation in failed back surgery syndrome.
Pain 1993;52:55-61.
- DeLissovoy G, et al. Cost-effectiveness of long-term intrathecal morphine
therapy for pain associated with
failed back surgery syndrome. Clin Ther 1997;19:96-112.
- Fager CA, Freidberg SR. Analysis of failures and poor results of lumbar
spine surgery. Spine 1980;5:87-94.
- Fritsch EW, et al. The failed back surgery syndrome: reasons, intraoperative
findings, and long-term results:
a report of 182 operative treatments. Spine 1996;21:626-33.
- Frymoyer JW and Cats-Baril WL. An overview of the incidence and costs
of low back pain.
Orthop Clin North Am 1991;22:263-71.
- Kidd D, et al. Spinal cord stimulation: an effective and cost-saving
treatment in the management of chronic pain.
In: Pain Treatment Centers at a Crossroads: A Practical
and Conceptual Reappraisal.
- Cohen M and Campbell J, eds. IASP Press: Seattle, 1996, p.176.
- Kumar K, et al. Epidural spinal cord stimulation for treatment of chronic
pain - some predictors of success.
A 15-year experience. Surg - Neurol 1998;50:110-21.
- Kumar K, et al. Treatment of chronic pain with spinal cord stimulation
versus alternative therapies:
cost-effectiveness analysis. Neurosurgery 2002;51:106-116.
- Kumar K, et al. Treatment of chronic pain by using intrathecal drug
therapy compared with conventional pain
therapies: a cost effectiveness analysis. J Neurosurg
2002;97(4):803-810.
- Long DM, et al. Clinical features of the failed-back syndrome. J Neurosurg
1988;69:61-71.
- Melzack R, Wall PD. Pain Mechanisms: a new theory. Science. 1965;150:971-9.
- North RB, et al. Failed back surgery syndrome: 5-year follow-up after
spinal cord stimulator implantation.
Neurosurgery 1991a; 28:692-9.
- North RB, et al. Failed back surgery syndrome: five-year follow up in
102 patients undergoing reoperation.
Neurosurgery 1991;28:685-91.
- North R, et al. Spinal cord stimulation for chronic, intractable pain:
experiences over two decades.
Neurosurgery 1993;32:384-95.
- North RB, Kidd DH. A prospective randomised study of spinal cord stimulation
versus re-operation for failed
back surgery syndrome: initial results. Stereotact
Funct Neurosurg 1994; 62:267-72.
- Ohnmeiss DD, Rashbaum RF. Patient satisfaction with spinal cord stimulation
for predominant complaints of
chronic, intractable low back pain.Spine J 2001;1:358-63.
- Paice J, et al. Intraspinal morphine for chronic pain: retrospective,
multicenter study.
J Pain Symptom Manag 1996;11:71-80.
- Papageorgiou AC, et al. Estimating the prevalence of low back pain in
the general population:
evidence from the South Manchester back pain survey.
Spine 1995;20:1889-94.
- Segal R, et al. Spinal cord stimulation revisited. Neurol Res 1998;
20:391-6.
- Spengler DM, et al. Low-back pain following multiple lumbar spine procedures:
failure of initial selection?
Spine 1980;5:356-60.
- Svensson HO, Andersson GBJ. The relationship of low-back pain, work
history, work environment, and stress:
a retrospective crosssectional study of 38
to 64 year old women. Spine 1989;14:517-22.
- Tutak U, et al. Intrathecal infusion systems of chronic low back and
leg pain of noncancer origin.
South Med J 1996;89:295-300.
- Winkelmuller M, et al. Long term effects of continuous intrathecal opioid
treatment in chronic pain of
nonmalignant etiology. J Neurosurg 1996;85:458- 467.
- Zucherman J, Schofferman J. Pathology of failed back surgery syndrome.
In: White AH, ed. Failed back surgery syndrome.Philadelphia:Hanley
& Belfus, 1986: 1-12.
|

|

|
|


