 |

|  | Therapy Information
Activa®Therapy
|
A second chance
at life
Parkinson's patients want a second chance. A second chance to enjoy the
things in life that Parkinson's disease can make so difficult.

Activa Parkinson's Control Therapy uses electrical stimulation to safely
and effectively treat some of the most disabling motor symptoms of Parkinson's
disease. As a result, many patients achieve greater control over their
body movements and get a second chance at life.
|
Now approved for advanced Parkinson's disease,
Activa Parkinson's Control Therapy is a safe and effective adjunctive treatment
option for many Parkinson's patients. Activa Therapy controls rigidity, bradykinesia/akinesia
and/or tremor - while reducing the duration of dyskinesia related to antiparkinsonian
medication. 1,2,3
References:
1. Data on file at Medtronic, Inc.
2. Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic
nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105-11.
3. The Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain
stimulation of the subthalamic nucleus or the pars interna of the globus pallidus
in Parkinson's disease. N Engl J Med. 2001;345:956-63.
4. Deuschl G, Fogel W, Hahne M et al. Deep brain stimulation for Parkinson's
disease. J Neurol 2002; 249:III/36-III/39
|
Advantages
of Activa® Parkinson's Control Therapy
Activa Therapy can provide more
than six additional hours of relief from the debilitating bradykinesia/akinesia,
rigidity and/or tremor of Parkinson's disease. It can also reduce the
duration of the dyskinesias that are a common side effect of Parkinson's
medications.1
Reversible
Unlike both pallidotomy and thalamotomy, Activa Therapy is a fully reversible,non-lesioning
,that does not damage brain tissue 2.3.4. The system may be
removed - preserving options for future therapies.
|
|
Adjustable
As the disease evolves, so will treatment requirements. The stimulation
parameters of the Activa Therapy neurostimulation system can be quickly
and easily adjusted by the physician and the patient (within physician set
limits) to maximize symptom control.
- N'Vision T is a pocket-sized, user
friendly, non invasive programmer for the management of all Medtronic
neuromodulation devices
- AccessT therapy patient controller
empowers patients with more control over their disease management (within
physician-set and locked parameters), reducing dependence on the medical
community 5
|
 |
Bilateral
Unlike Pallidotomy and thalamotomy, Activa therapy, via a single KinetraT
neurostimulator, can be implanted to treat unilateral and expanded to bilateral
treatment as dictated by disease evolution. 6,7,8
Activa therapy
has a reassuring safety profile
- Activa therapy is well tolerated - the majority
of side-effects are usually mild and transient in nature 6,9,
and can often be easily managed by adjusting the stimulation parameters 2
- The majority of patients recover quickly and
experience little discomfort during the healing process
- The risk of potential surgical complications
with Activa Therapy is similar to those encountered with other stereotactic
neurosurgical procedures 6,10,11
- There is no damage to the brain tissue as a
result of Activa Therapy
- Activa Therapy has been shown to be cognitively
safe 12, 13
References:
1 Data on file at Medtronic, Inc.
2 Pollak P, Fraix V, Krack P et al. treatment results: Parkinson's disease.
Mov Disord 2002; 17:S75-
3 Ardouin C, Pillon B, Peiffer E, et al. Bilateral subthalamic or pallidal
stiumulation for Parkinson's
4 Haberler C, Alesch F, Mazal PR, et al. No tissue damage by chronic
deep brain stimulation in Parkinson's disease. Ann Neurol. 2000; 48:372-376.
5 Vesper J, Chabardes S, Fraix V et al. Dual channel deep brain stimulation
system (kinetra) for Parkinson's disease and essential tremor: a prospective
multicentre open label clinical study. J Neurol Neurosurg Psychiatry 2002 ;
73 :275-280
6 Limousin P, Speelman JD, Gielen F et al. Multicentre European study
of thalamic stumulation in parkinsonian adn essential tremor. J Neurol Neurosurg
Psychiatry 1999; 66: 289-296
7 Medtronic Global clinical study report : Activa Parkinson's disease control
therapy (N1-3169). 1999
8 The Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain
stimulation of the subthalamic nucleus or the pars interna of the globus pallidus
in Parkinson's disease. N Engl J Med. 2001;345:956-63.
9 Molinuevo JL, Valldeoriola F, Tolosa E et al. Levodopa withdrawal after
bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch
Neurol 2000; 57: 983-988
10 Deuschl G, Fogel W, Hahne M et al. Deep brain stimulation for Parkinson's
disease. J Neurol 2002; 249:III/36-III/39
11 Schuurman PR, Bosch DA, Bosch DA, Bossuyt PMM et al. A comparison
of continuous thalamic stimulation and thalamotomy for suppression of severe
tremor. New Eng J Med 2000; 342: 461-468
12 Ardouin C, Pillon B, Peiffer E, Benabid AL et al. Bilateral subthalamic or pallidal stimulation for Parkinson's disease affects neither memory nor executive functions: a consecutive series of 62 patients. Ann Neurol 1999; 46: 217-223
13 Troster Al, Pahwa R et al.Neuropsychological and quality outcome after
thalamic stimulation for essential tremor. Neurology 1999; 53: 1774 - 1780
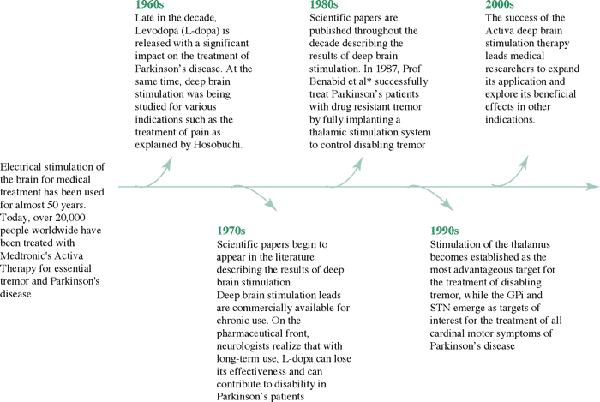
History of Deep
Brain Stimulation
Neurologists and neurosurgeons have used electrical stimulation since the 1960s
as a way to locate and distinguish specific sites in the brain. During the process,
they discovered that stimulation of certain brain structures suppresses the
symptoms of neurological disorders such as essential tremor and Parkinson's
disease. Medtronic developed brain stimulation technology in the 1980s in conjunction
with leading physician researchers. In 1987, professors Alim-Louis Benabid and
Pierre Pollak of the University of Grenoble in France published the results
of the first application of chronic deep brain stimulation for the treatment
of movement disorders.
- Activa Parkinson's Control Therapy, which has been approved in Canada, Europe and Australia since 1998 and in the United States since January 2002, extends the use of Medtronic's brain stimulation
technology to benefit patients with advanced, levodopa-responsive Parkinson's
disease. Activa Parkinson's Control Therapy targets the subthalamic nucleus
(STN) or the globus pallidus interna (GPi) to suppress some of the disabling
symptoms of Parkinson's disease.
- Activa Tremor Control Therapy, which has been
approved in Canada, Europe and Australia since 1995 and in the United States
since 1997, targets the ventral intermediate nucleus (VIM) of the thalamus
to suppress tremor associated with essential tremor or Parkinson's disease.
The History of Deep
Brain Stimulation for the Treatment of Movement Disorders
Electrical stimulation of the brain has been used since 1987 to treat movement
disorders. Today over 20000 people worldwide have benefited from the Medtronic
deep brain stimulation technology called Activa Therapy for essential tremor
and advanced Parkinson's disease.
* Benabid AL, Pollak P, Louveau
A et al. Combined thalamotomy and stimulation stereotactic surgery of the VIM
thalamic nucleus for bilateral Parkinson's disease. Appl Neurophysiol 1987;
50: 344-346
|


