New opportunities to
redefine the patient experience
93% smaller than modern-day pacemakers5
- Increased patient satisfaction
- No chest scar
- No bump
- No visible or physical reminder of the pacemaker under the skin
- Fewer post-implant restrictions
Reduce complications associated with traditional pacing technology
Pocket Related Complications
- Infection
- Hematoma
- Erosion
Lead Related Complications
- Fractures
- Insulation breaches
- Venous thrombosis and obstruction
- Tricuspid regurgitation
The Micra Transcatheter Pacing System is composed of three elements:
- Micra Cardiocapsule
- Micra Delivery Catheter
- Micra Introducer

Micra Specifications
|
Volume
|
0.8 cc
|
|
Length
|
25.9 mm
|
|
Outer Diameter
|
6.7 mm (20.1 Fr)
|
|
Mass
|
2.0 g
|
|
Pacing Mode
|
VVIR
|
|
Longevity
|
10 years (100% VP, 1.5V, 60 bpm, 500 ohms, 0.24 ms)
|
FlexFix™ Nitinol Tines

Provides atraumatic and secure capsule placement6
- Multidimensional redundancy, two tines have 15 times the holding force necessary to hold the device in place7
- Designed to minimize tissue damage during deployment, repositioning and retrieval8
- Optimal electrode tissue interface allows for low and stable chronic thresholds9
Device life cycle management options
- Micra is designed to be programmed off at the end of service and can be differentiated from additional Micra devices, if subsequent devices are implanted
- The Micra design incorporates a proximal retrieval feature to enable acute retrieval
- Successful retrieval demonstrated after 28 months in chronic animal models10
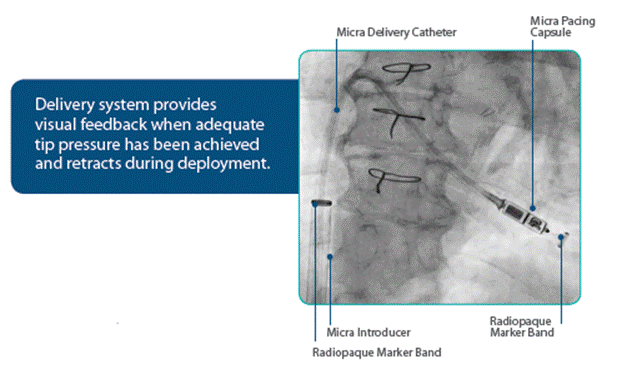
Micra Deliver Catheter
- Integrated delivery system facilitates a streamlined implant procedure
- 105 cm long catheter system with a handle that controls deflection and deployment of the Micra Pacing Capsule11

Micra Introducer
Lubricious hydrophilic coating facilitates smooth vessel navigation.12
Introducer features:
- 23 Fr inner diameter (27 Fr outer diameter)
- Hydrophilic coated sheath
- Silicone oil coated dilator tip
For training materials and additional information about Micra, please visit Micra TPS Academy.
See the device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential adverse events.
References
- Williams, Eric, Whiting Jon; Micra Transcatheter Pacing System Size Comparison, November 2014, Medtronic Data on File.
- Steinwender C, et al. Early Electrical Performance of a Novel Leadless Transcatheter Pacemaker System: Data from the Micra Clinical Study, Presented at HRS 2015 (P009-29)
- Reynolds D, Duray GZ, Omar R, et al. A Leadless Intracardiac Transcatheter Pacing System. N Eng J Med. Published online November 9, 2015
* Based on 300 patients @ 6 months: 1.1 V, 50% Pacing, 594 Ω 72 bpm, 0.24 ms (average settings)
- Medtronic Micra MC1VR01 Clinician Manual, November 2014.
- Williams, Eric, Whiting Jon; Micra Transcatheter Pacing System Size Comparison, November 2014, Medtronic Data on File.
- Eggen, Mike. FlexFix Tine Design. April 2015. Medtronic Data on File.
- Grubac V, Goff R, Rys K, Eggen M, Bonner M, Nikolski V. Analysis of the Micra fixation mechanism use conditions and holding energy requirements. Presented at EHRA Europace 2014 (Abstract 16-56).
- Eggen, Mike. FlexFix Tine Design. April 2015. Medtronic Data on File.
- Bonner MD, Eggen M, Hilpisch K, et al. Performance of the Medtronic Micra Transcatheter Pacemaker in a GLP Study. Heart Rhythm. May 2014;11(5):S19.
- Bonner MD, Neafus N, Byrd CL, et al. Extraction of the Micra transcatheter pacemaker system. Heart Rhythm. May 2014;11(5):S342.
- Medtronic Micra MC1VR01 Clinician Manual, November 2014.
- Medtronic Micra MC1VR01 Clinician Manual, November 2014.
|






